Get competitive Bids from Pre-Vetted Vendors withAutomated RFP Creation & One-Click Publishing®
Publish RFP function: publish your RFP so all potential CROs and Service Providers can see it
Be first to benefit from this brand-new product
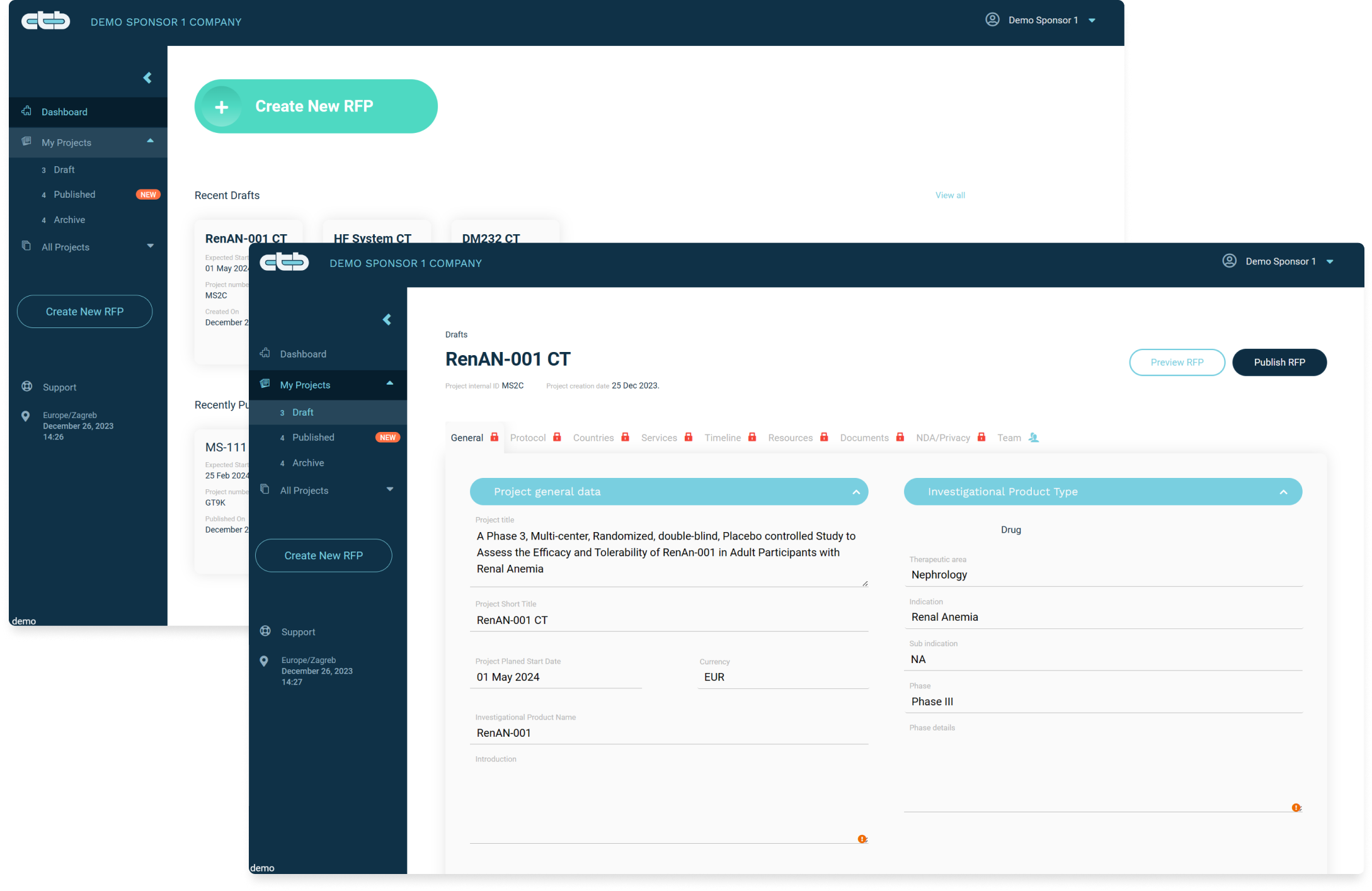
Publish RFP function: publish your RFP so all potential CROs and Service Providers can see it

A minimum of 80% reduction in time and money spent on RFP creation, CROs and Service Providers discovery, and proposal selection processes
RFP Process
Streamline your RFP creation with our automated AI enhanced tools. Save time and focus on what matters most – your project’s success.
The Automated RFP process for sponsors in the Clinical Trials Tenders Hub (CTB) is a streamlined, technology-driven approach for creating, and publishing RFPs for clinical trials. This feature is designed to expedite the process of soliciting and receiving proposals from Contract Research Organizations (CROs) and Service Providers.

RFP Process
Once an RFP is ready, Sponsor can publish it. This is when the project becomes an actual tender, and it gains access to a diverse pool of CROs and Service Providers, spanning various specializations and geographic locations. This increases the chances of finding the right partner for specific needs. Sponsors benefit from a system of ratings and reviews, understanding the performance and reliability of CROs and Service Providers based on historical data and feedback from peers.

Broad visibility through RFP publishing on the Clinical Trials Tenders Hub (CTB) ensures sponsors’ trial opportunities reach a wide, diverse audience of CROs and Service Providers globally. This feature increases competitive bidding and quality of proposals by attracting various specialized and capable CROs. It enhances the chances of finding the right match for specific trial needs, leading to more efficient, effective clinical trial partnerships and outcomes.

Customizable Information in CTB allows sponsors to control the disclosure of trial details in the RFP view within available folder for CROs. Sponsors can tailor the amount and type of information shared, balancing transparency with confidentiality. This feature helps protect sensitive data before NDA execution, while providing sufficient detail to attract suitable CROs.

Automated NDA Negotiation in CTB streamlines the process of establishing confidentiality agreements between sponsors and CROs and Service Providers. It uses templates and predefined terms to expedite discussions, reduce manual efforts, and ensure secure information exchange. This feature allows for quicker commencement of detailed proposal discussions and helps maintain the integrity and confidentiality of sensitive trial information throughout the RFP process.

Post-NDA Proposal Receipt in CTB refers to the stage where sponsors receive detailed proposals from CROs and Service Providers after Non-Disclosure Agreements (NDAs) are in place. This ensures that sensitive trial information is protected until confidentiality is assured. It facilitates a secure exchange of comprehensive proposals, allowing sponsors to assess the capabilities and offers of CROs and Service Providers under the protection of legally binding confidentiality, leading to informed decision-making for trial partnerships.

The Uniform Proposal Format in CTB standardizes the structure and content of proposals submitted by CROs and Service Providers. This consistency allows sponsors to easily compare and evaluate different proposals on a like-for-like basis. It streamlines the review process, reduces ambiguity, and enhances decision-making efficiency. By ensuring all critical information is presented clearly and uniformly, it enables faster, more accurate assessment and selection of the best suited CROs and Service Providers for clinical trials.

In-App Project Communication in CTB enables direct, secure, and efficient dialogue between sponsors and CROs and Service Providers within the platform. This feature supports real-time messaging, updates, and collaboration, ensuring all communications are centralized and easily accessible. It enhances coordination, speeds up decision-making, and maintains a clear record of discussions, aiding in transparency and project management throughout the clinical trial process, from initial RFP to final selection.
Bid Meeting Invitations in CTB are notifications sent to CROs and Service Providers encouraging them to participate in a bidding meeting for a specific RFP. This feature facilitates direct interaction between sponsors and interested CROs and Service Providers, allowing for live discussions, Q&A sessions, and clarifications about the trial requirements. It enhances the bidding process, fosters better understanding, and allows CROs and Service Providers to tailor their proposals more effectively, leading to more informed and competitive bids.

Final CRO Selection Notification in CTB is the formal communication sent to all participating CROs, announcing the sponsor’s decision at the end of the bidding process. It informs the chosen CROs of their successful bid and provides feedback or thanks to others for their participation. This transparency ensures all parties are promptly notified of the outcome, allowing them to proceed with the next steps or seek other opportunities, maintaining clarity and respect throughout the process.

Accelerated CROs and Service Providers Selection in CTB is facilitated by streamlined RFP processes, uniform proposal formats, and efficient communication tools. The platform’s features enable sponsors to quickly review, compare, and decide on the best-suited CROs and Service Providers for their trials. This rapid selection process reduces the time from RFP to trial initiation, allowing for faster commencement of critical research, ensuring timely trial progression, and bringing therapies to market more quickly.
Time and Cost Efficiency in CTB is achieved through streamlined processes, automation, and centralized communication, significantly reducing the duration and expense of the clinical trial setup. By automating RFPs, standardizing proposals, and facilitating direct negotiations, CTB minimizes administrative burdens and speeds decision-making. This efficiency leads to quicker trial starts, reduced overhead costs, and better allocation of resources, benefiting both sponsors, CROs and Service Providers.
No membership fee, no hidden costs, just benefits and support.
The time you save at the very beginning of the project gives your product a chance to reach the market in a record time, much before your competitors.